43 multi step reaction energy diagram
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product. CH 368: Unit 2 - University of Texas at Austin Conventionally, they are a plot of energy (in the rigorous case, free energy, G) versus the reaction coordinate. 6. Reaction Mechanism. The mechanism of a reaction is a stepwise description of how reactants are converted to products. Of course, some reactions occur in a single step, as represented in the reaction path diagram above. Such ...
Multi-Step Reaction - an overview | ScienceDirect Topics The multi-step reaction catalyzed by the PDH complex involves sequential reactions with the intermediates being relayed between the subunits via the flexible lipoyl domains. E1 catalyzes the decarboxylation of pyruvate forming 2- (1-hydroxyethyldiene)-TPP bound to E1 (TPP is thiamin pyrophosphate).

Multi step reaction energy diagram
Energy Diagrams of Reactions | Fiveable To find the activation energy, you should be looking for two numbers: the potential energy of the reactants and the energy of the activated complex (the maximum point). (energy of activation complex) - (PEreactants) (100 kJ) - (40 kJ) = 60 kJ In other words, it takes 60 kJ of energy to complete the reaction. Energetics: 4.32 - Hess' law energy cycles and diagrams An energy cycle is a diagram showing how three, or more reactions are interconvertable. Energy cycles can be constructed to find unknown energy changes, by adding up the energies of steps from a different route to the same final products. For example, if the energy of the conversion A B is known, and C can also be converted into B, C B, and ... Potential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
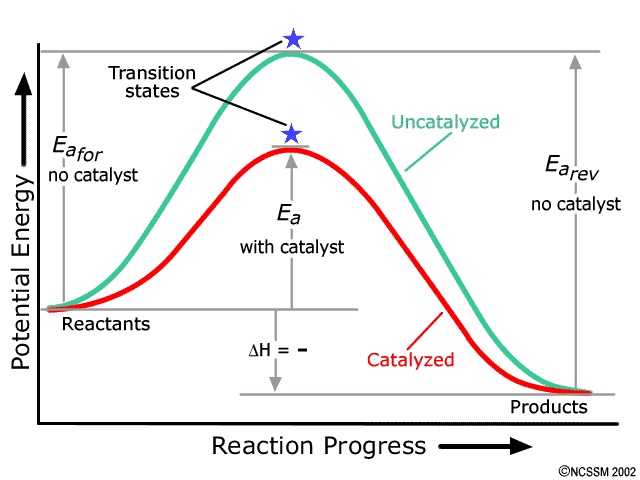
Multi step reaction energy diagram. ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide This diagram shows that, overall, the reaction is exothermic. The products have a lower energy than the reactants, and so energy is released when the reaction happens. It also shows that the molecules have to possess enough energy (called activation energy) to get the reactants over what we think of as the "activation energy barrier". Ch 16 Pt 2 Smartbook Flashcards - Quizlet The reaction NO2 (g) + CO (g) → NO (g) + CO2 (g) has ΔH°overall = -226 kJ/mol. A proposed reaction mechanism is shown. Choose the statement (s) that accurately describe the reaction energy diagram for the above reaction. -There will be three peaks. -The Ea of the first step will be larger than the second or third step. 12.7 Catalysis - Chemistry This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ... E1 Reaction Mechanism and E1 Practice Problems - Chemistry Steps Step 1: Loss of he leaving group. The energy diagram of the E1 mechanism demonstrates the loss of the leaving group as the slow step with the higher activation energy barrier: The dotted lines in the transition state indicate a partially broken C-Br bond. The Br being the more electronegative element is partially negatively charged and the ...
Reaction Mechanisms and Multistep Reactions - Chemistry LibreTexts Multi-step (Consecutive) Reactions Mechanisms in which one elementary step is followed by another are very common. (step 1) A + B → Q (step 2) B + Q → C (net reaction) A + 2 B → C (As must always be the case, the net reaction is just the sum of its elementary steps.) TIGER - NCSSM Distance Education and Extended Programs This diagram shows a potential energy diagram for a multi-step mechanism. NOplusH2_ver_1-640.gif This diagram shows a one-step mechanism for a reaction. NOplusH2_ver_2-640.gif This diagram shows a two-step mechanism for a reaction with the first step being rate determining. NOplusH2_ver_3-640.gif This diagram shows a two-step mechanism for a ... From the given energy diagram - step of the mechanism, ΔG , Ea and Keq ... Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram. Energy Diagrams: Describing Chemical Reactions Self-test question #1 Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn, as well as DG 1 * and DG 2 * for the first and second activation energies, respectively.
Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1 Reaction Mechanisms - Introductory Chemistry - 1st Canadian Edition A potential energy diagram for this multi-step reaction can be drawn as shown in Figure 17.12 "Multi-step Reaction Potential Energy Diagram." Notice each step has its own activation energy, and transition state (or activated complex ), which is the highest-energy transitional point in the elementary step. How do you find the rate determining step from a graph? | Socratic It is characterized by its high activation energy. Explanation: The rate determining step in a reaction mechanism is the slowest step. It is characterized by its high activation energy. Consider the energy diagram represented below of a two-step mechanism. The first step is the slow step since it has the highest activation energy. Solved Label the following multi-step reaction energy - Chegg Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (10 ratings) Transcribed image text: Label the following multi-step reaction energy diagram AHn> 0 Reaction Intermediate Ealstep 2) Products Ea (step 1) Reactants Reaction progress.
Arrhenius Theory and Reaction Coordinates - Chemistry 302 The key is that there are many many potential paths between reactants and products. The reaction coordinate represents the lowest energy path. For example, in the reaction of CH 3 Cl + OH- to form CH 3 OH and Cl-, the mechanism of this reaction is a single step in which the CH 3 Cl collides with the OH- and forms the products. We can envision a ...
Chem 51A. Lecture 19. Organic Chemistry: Ch. 6. Energy Diagrams ... -11:11 Energy Diagram for Single Step Reaction-17:13 Transition State-26:06 Energy Diagram for Multi-Step Reaction-34:46 Steps in the Multi-Step Reaction-30:01 Reverse Reaction of Multi-Step Reaction-41:34 Rates of Reaction-47:15 Other Factors Affecting Rate. Required attribution ...
Answered: Sketch an energy diagram for a two-step… | bartleby Con-sider this one-step reaction for the formation of this compound:NO (g)+Cl₂ (g) →NOCl (g)u0007+Cl (g) ΔH°u0006=83 kJ (a) Draw a reaction energy diagram, given Ea (fwd)is 86 kJ/mol. (b) Calculate Ea (rev). (c) Sketch a possible transition state for the reaction. arrow_forward 10.
Solved 3. The energy diagram for a multi-step reaction | Chegg.com The energy diagram for a multi-step reaction mechanism for the decomposition of C4H9Br is shown below.
7.4 SN1 Reaction Mechanism, Energy Diagram and Stereochemistry Because S N 1 is a multiple-step reaction, so the diagram has multiple curves, with each step can be represented by one curve. Out of the three steps, the activation energy for step 1 is the highest, therefore step 1 is the slowest step, that is the rate-determining step. Figure 7.4a Energy diagram for SN1 reaction between (CH3)3CBr and H2O
Reaction mechanism and rate law (article) | Khan Academy A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. The slowest step in a reaction mechanism is known as the rate-determining step.
Energy Diagram Module Series- Part Three: Intermediates and Rate ... Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism. You will soon see most reactions proceed in a multistep fashion. In this case, reaction mechanisms often form lower energy and sometimes isolatable intermediates.
Multistep reaction energy profiles (video) - Khan Academy Next, let's look at the energy profile for this multi-step reaction. Energy profiles usually have potential energy in the y-axis and then reaction progress on the x-axis. So as we move to the right on the x-axis, the reaction is occurring. This first line on our energy profile represents the energy level of our reactants, which are BC and D.
Reaction Mechanisms - Chem1 2 Multi-step (consecutive) reactions. Mechanisms in which one elementary step is followed by another are very common. step 1: A + B → Q. step 2: B + Q → C. net reaction: A + 2B → C. (As must always be the case, the net reaction is just the sum of its elementary steps.) In this example, the species Q is an intermediate, usually an unstable ...



Post a Comment for "43 multi step reaction energy diagram"