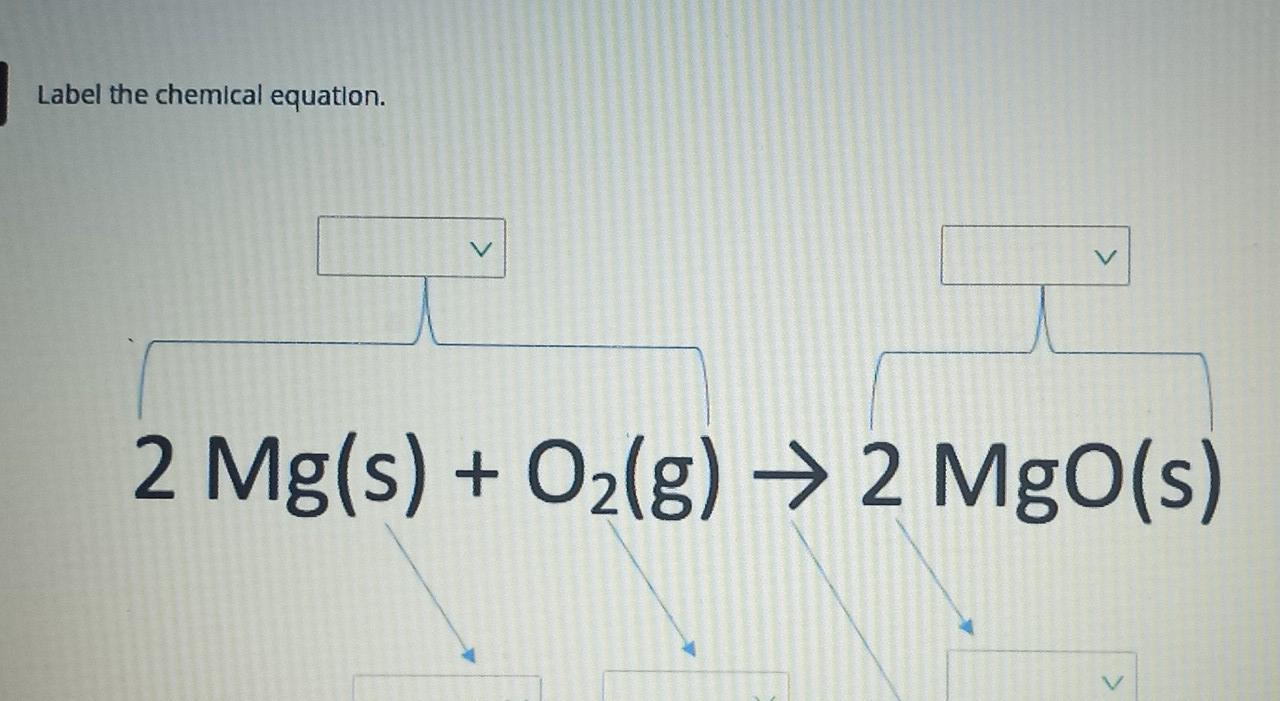
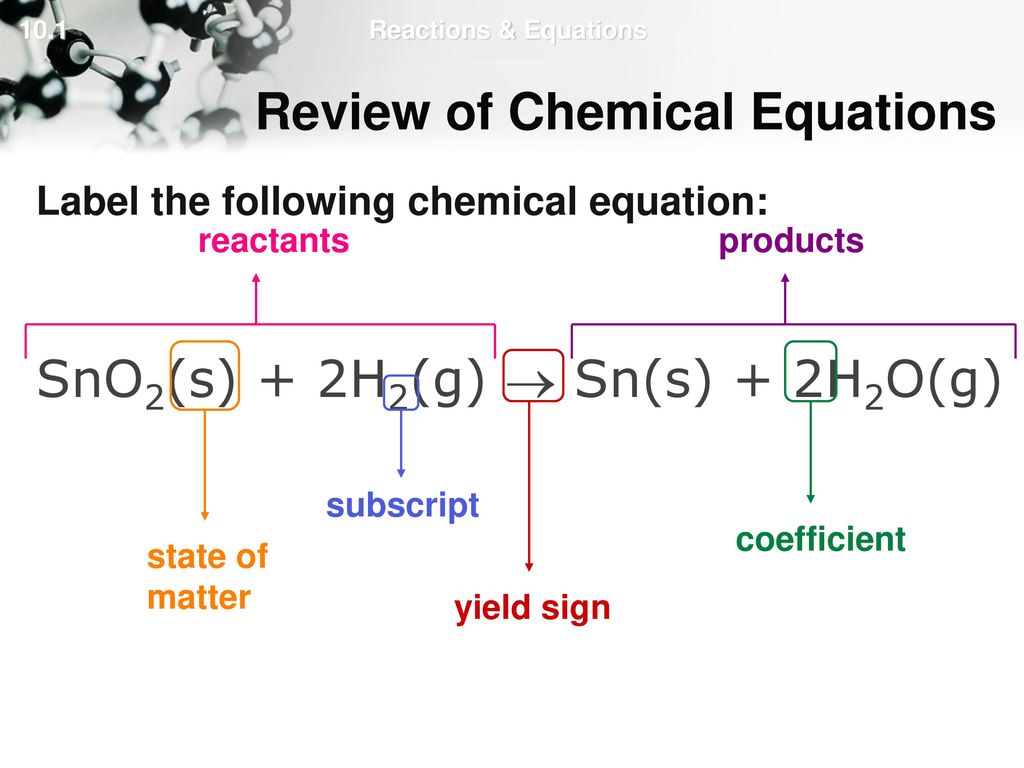
45 chemical equation with labels
Laebling A Chemical Equation Part I - YouTube Aug 5, 2012 ... Labeling chemical equations and identifing reactants, yield and products. 4.1: Writing and Balancing Chemical Equations Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation form: CO 2(aq) + NaOH(aq) → Na 2CO 3(aq) + H 2O(l) Balance is achieved easily in this case by changing the coefficient for NaOH to 2, resulting in the molecular equation for this reaction: CO 2(aq) + 2 NaOH(aq) → Na 2CO 3(aq) + H 2O(l)
7.3: Chemical Equations - Chemistry LibreTexts We should also be able to read a chemical equation and put into words a description for a chemical reaction. Consider, Cu(s) + AgNO 3(aq) → Cu(NO 3) 2(aq) + Ag(s) This equation may be described something like Solid copper reacts with an aqueous solution of silver nitrate to produce an aqueous solution of copper (II) nitrate and solid silver metal.
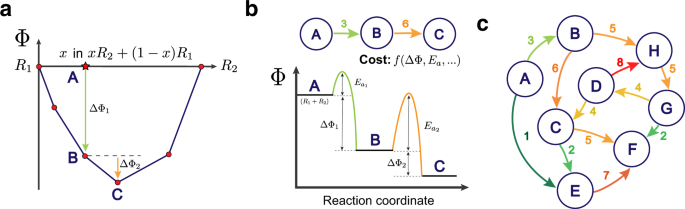
Chemical equation with labels
How would you label each formula in the chemical equation below ... Jan 23, 2017 ... We designate the reactants as written........ The reactants are on the LEFT HAND SIDE of the equation. The products are on the right hand ... Examples of Balanced Chemical Equations - ThoughtCo Writing balanced chemical equations is essential for chemistry class.Here are examples of balanced equations you can review or use for homework. Note that if you have "1" of something, it does not get a coefficient or subscript. The word equations for a few of these reactions have been provided, though most likely you'll be asked to provide only the standard chemical equations. The Chemical Equation, Its Parts, Labels and State Symbols Jan 8, 2013 ... The chemical equation is a shorthand way of describing a chemical reaction. A chemical equation may consist of simple formulas or very complex, ...
Chemical equation with labels. What is a Chemical Equation? - Definition & Examples A chemical equation provides information about the correct proportions of ingredients that are needed to make new substances in chemical reactions. To make a large glass of lemonade, use 1 1/2 ... Label each reactant and product in the given chemical reaction. Write the products for the given sequence of the reaction. Predict the product or reactant in the depicted reaction. If there is more than one product, circle ... Align and label in chemical equation - TeX - LaTeX Stack Exchange Apr 17, 2017 ... For the first objective, change from an align* to an align environment and use \notag directives on lines 1, 4, and 5. 4.E: Chemical Reactions and Equations (Exercises) Write and balance the chemical equation described by Exercise 3. Write and balance the chemical equation described by Exercise 4. The formula for propane is C 3 H 8. Balance: ___NaClO 3 → ___NaCl + ___O 2 Balance: ___N 2 + ___H 2 → ___N 2 H 4 Balance: ___Al + ___O 2 → ___Al 2 O 3 Balance: ___C 2 H 4 + ___O 2 → ___CO 2 + ___H 2 O
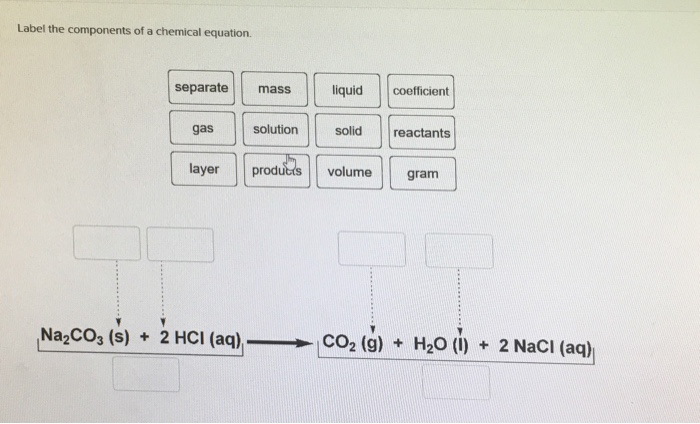
For the chemical equations shown below, label each reactant as ... Conjugate acid-base pairs. Conjugate Acid-Base. As shown by the labels in the figure: Reaction 1: HPO2−4(aq)+H2O(l)⇌H2PO−4(aq)+OH−(aq) H P O 4 2 − ( a ... Definition of dissolve and the state label (aq) in chemical equations A chemical equation is not meant to convey everything. It is just a shorthand notation to describe chemical reactions. A real experiment or a research paper should describe the experimental condition with sufficient details in such a way that an experienced person should be able to repeat it. 3.1: Chemical Equations - Chemistry LibreTexts The initial (unbalanced) equation is as follows: Ca 5(PO 4) 3(OH)(s) + H 3PO 4(aq) + H 2O ( l) → Ca(H 2PO 4) 2 ⋅ H 2O ( s) 1. B Identify the most complex substance. We start by assuming that only one molecule or formula unit of the most complex substance, Ca 5(PO 4) 3(OH), appears in the balanced chemical equation. 2. Chapter 4 Quantities of reactants and products Phase labels: letters written in parenthesis after a reactant or product to indicate whether the substance is a solid (s), liquid (l), gas (g) or dissolved in ...
How do you Write a Chemical Equation? - A Plus Topper Step 2: Thus, the word equation is Magnesium + Oxygen Magnesium oxide Step 3: Replacing the names with symbols and formulae, we get the chemical equation as Mg + O 2 MgO Step 4: The number of atoms of the elements are To balance oxygen on both sides, multiply RHS by 2, i.e., Mg + O 2 2MgO 7.4: How to Write Balanced Chemical Equations Steps in Balancing a Chemical Equation Identify the most complex substance. Beginning with that substance, choose an element(s) that appears in only one reactant and one product, if possible. Adjust the coefficients to obtain the same number of atoms of this element(s) on both sides. 7.3: The Chemical Equation - Chemistry LibreTexts Jul 18, 2022 ... Reactants and Products ... To describe a chemical reaction, we need to indicate what substances are present at the beginning and what substances ... How to Write a Chemical Equation (with Pictures) - wikiHow If you want to write a chemical equation, start by writing the chemical formulas of each reactant. Use the prefixes, such as mono-, di-, tri-, and tetra-, to figure out the number of atoms present for each element, and write this number as a subscript for each element. For example, dihydrogen monoxide would be more easily written as H2O.
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example: 2NaHCO 3 (s) → 200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O (ℓ) Key Takeaways
5.2 Chemical Equations - Lumen Learning If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H 2 (g) + O 2 (g) → H 2 O(ℓ) Note. ... To make this chemical equation conform to the law of conservation of matter, we must revise the amounts of the reactants and the products as necessary to get the same ...
Answered: Please convert each word equation to a… | bartleby ASK AN EXPERT. Science Chemistry Please convert each word equation to a chemical equation (including all phase labels) • Reaction 3: Copper (II) hydroxide decomposes upon exposure to heat to yield cupric oxide and water. Cupric oxide is a black solid. What can you do to be sure all the copper (II) hydroxide has been converted to cupric oxide ...
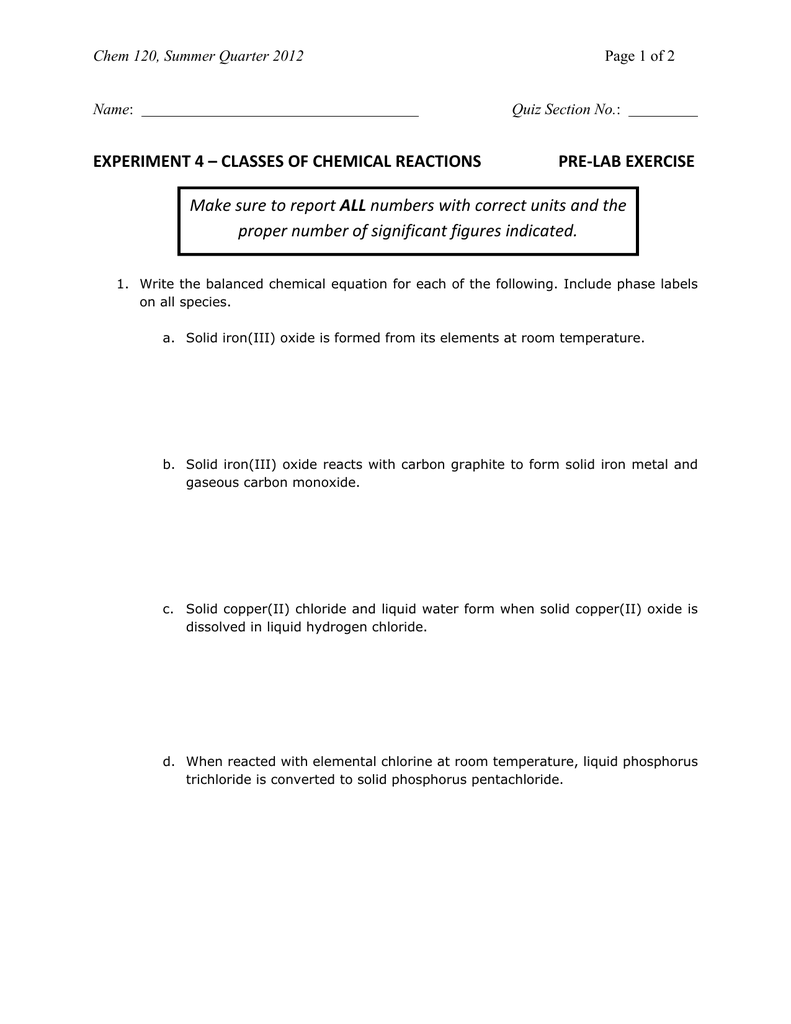
Solved Pre-Lab Ouestions 1. Write a balanced chemical | Chegg.com Question: Pre-Lab Ouestions 1. Write a balanced chemical equation including phase labels for the reaction between aqueous copper (II) nitrate and aqueous sodium hydroxide 2. Nitrogen monoxide (NO) and nitrogen dioxide (NO2) are toxic, corrosive gases that significantly lower blood pressure when inhaled.
Label the chemical Equation - Liveworksheets Label the chemical Equation worksheet Live worksheets > English Label the chemical Equation Label the parts of the chemical Equation ID: 1570824 Language: English School subject: Science Grade/level: 5 Age: 7-9 Main content: Chemical Equation Other contents: NA Add to my workbooks (2) Download file pdf Embed in my website or blog
Chemical Equation Balancer Practice by balancing a few of the equations below. If you get stuck, click the links to use our chemical equation balance calculator to see the balanced result and the four easy steps to get there: Aluminium + Sodium Hydroxide + Water = Sodium Aluminate + Hydrogen Gas: Al + NaOH + H2O = NaAlO2 + H2.
What are Chemical Equations? Detailed Explanation, Examples - BYJU'S A few examples of chemical equations are listed in bulleted text below. PCl5 + 4H2O → H3PO4 + 5HCl SnO2 + 2H2 → 2H2O + Sn TiCl4 + 2H2O → TiO2 + 4HCl H3PO4 + 3KOH → K3PO4 + 3H2O Na2S + 2AgI → 2NaI + Ag2S How do you write chemical formulas? There's a particular way of writing what's in a molecule called a chemical formula.
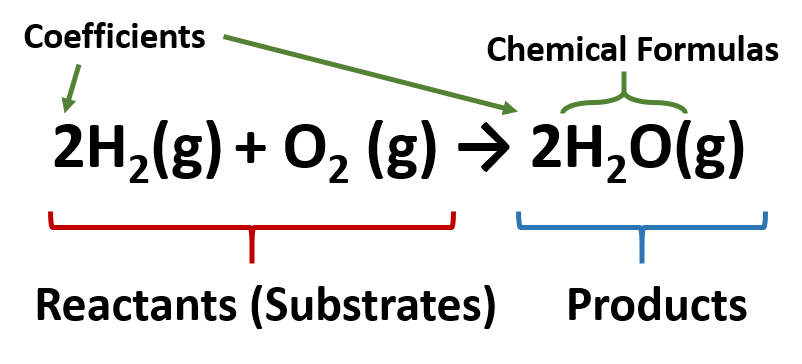
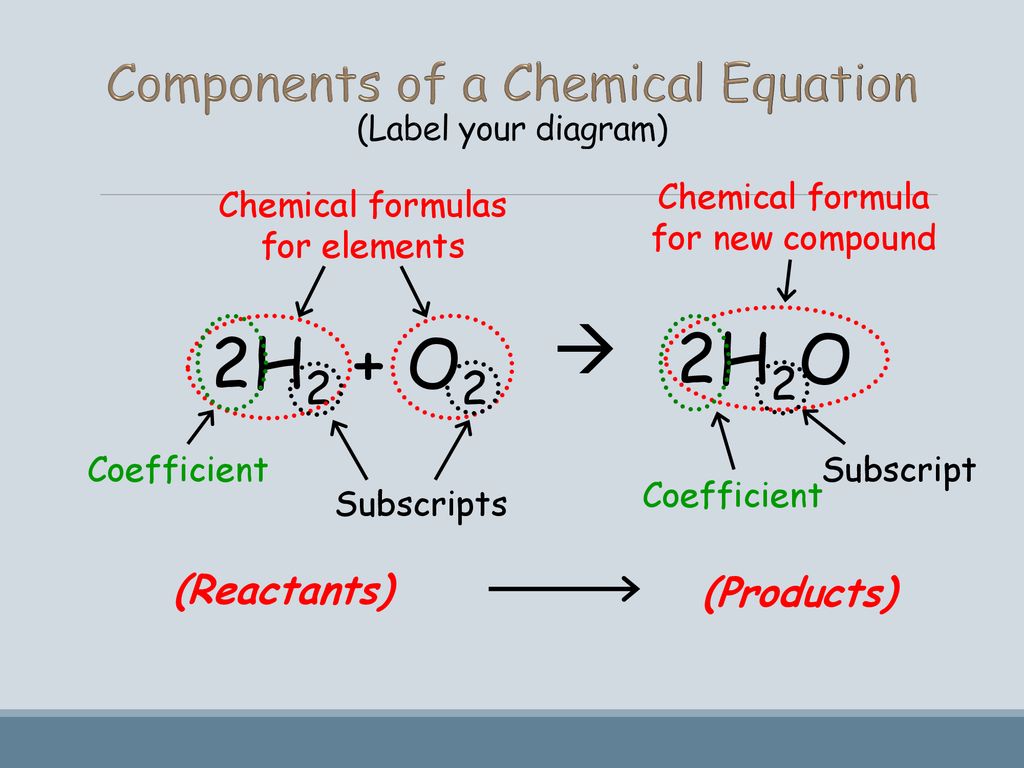
5.3: Chemical Equations - Medicine LibreTexts \[\ce{H_2 + O_2 \rightarrow H_2O} \label{Eq1} \] This statement is one example of a chemical equation, an abbreviated way of using symbols to represent a chemical change. The substances on the left side of the arrow are called reactants, and the substances on the right side of the arrow are called products.
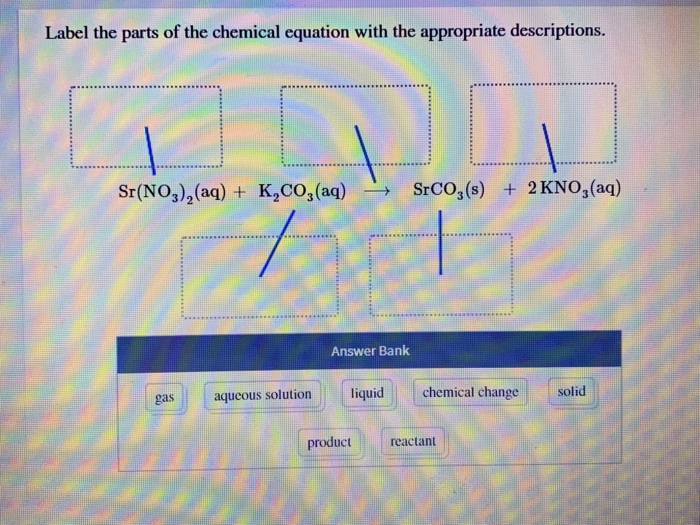
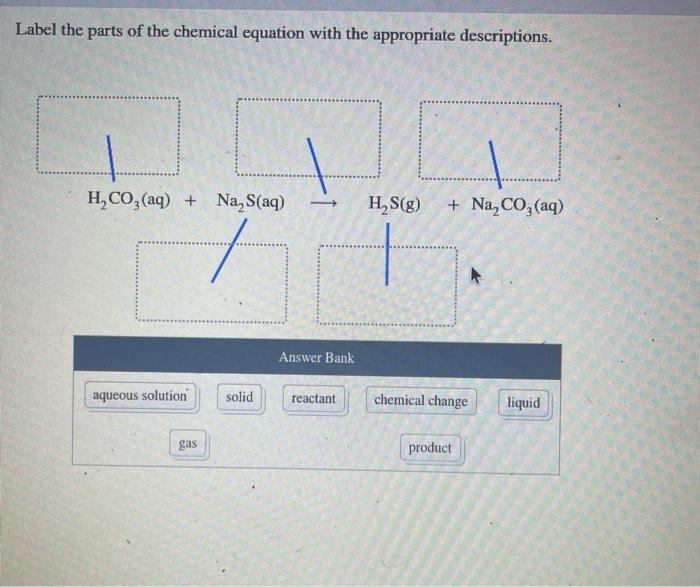
Label the parts of the chemical equation with the appropriate ... Nov 14, 2020 ... Label the parts of the chemical equation with the appropriate descriptions. Sr(NO3)2(aq) + K2CO3(aq) ⟶ SrCO3(s) + 2KNO3(aq). answer bank-.
Labeling A Chemical Equation Part 2 - YouTube Aug 5, 2012 ... Calculating Acceleration · Balancing Redox Using Half-Reaction Method in Acidic Solution | Learn Chemistry with Ma'am Cess · Exercise of Chemical ...
3 Steps for Balancing Chemical Equations - ThoughtCo A chemical equation describes what happens in a chemical reaction.The equation identifies the reactants (starting materials) and products (resulting substances), the formulas of the participants, the phases of the participants (solid, liquid, gas), the direction of the chemical reaction, and the amount of each substance. Chemical equations are balanced for mass and charge, meaning the number ...
7.3: Chemical Equations - Chemistry LibreTexts Use the common symbols, ( s), ( l), ( g), ( a q), and → appropriately when writing a chemical reaction. In a chemical change, new substances are formed. In order for this to occur, the chemical bonds of the substances break, and the atoms that compose them separate and rearrange themselves into new substances with new chemical bonds.
The Chemical Equation, Its Parts, Labels and State Symbols Jan 8, 2013 ... The chemical equation is a shorthand way of describing a chemical reaction. A chemical equation may consist of simple formulas or very complex, ...
Examples of Balanced Chemical Equations - ThoughtCo Writing balanced chemical equations is essential for chemistry class.Here are examples of balanced equations you can review or use for homework. Note that if you have "1" of something, it does not get a coefficient or subscript. The word equations for a few of these reactions have been provided, though most likely you'll be asked to provide only the standard chemical equations.
How would you label each formula in the chemical equation below ... Jan 23, 2017 ... We designate the reactants as written........ The reactants are on the LEFT HAND SIDE of the equation. The products are on the right hand ...





















Post a Comment for "45 chemical equation with labels"